BMAC (Bone Marrow Aspirate Concentrate)
Orthopedic minimally invasive procedure to collect bone marrow aspirate containing anti-inflammatory proteins and grown factors.
Regenerative Therapy
Bone Marrow Aspirate Concentrate (BMAC) is an orthopedic therapeutic procedure that involves collection of your own bone marrow elements and then injecting them into a site of injury, pain, or damage. BMAC devices are one of the few forms of harvesting devices currently cleared by the FDA. The stem cells contained in BMAC are just one portion of the contents, and are obtained from your own bone marrow.
What Elements are Included in BMAC?
The bone marrow is most often obtained by aspiration of the iliac crest (part of the pelvis) and includes mesenchymal stem cells (MSCs), growth factors, and anti-inflammatory cytokines as well as other blood products and regenerative cells.
Which Conditions can be Treated by BMAC?
BMAC treatment or orthopedic conditions is still considered experimental. Recent studies have shown good to excellent outcomes for treatment of mild to moderate osteoarthritis of the knee (OA) and limited defects in the knee cartilage. Other conditions studied include tendon injuries, bone fractures, and bone death (osteonecrosis or AVN).
Consider Your Treatment Options
conditions studied for
BMAC Ortho Injections
Mild to Moderate Knee Arthritis
A recent systematic review has demonstrated that BMAC ortho injections are effective in improving pain and patient-reported outcomes (PROs) in patients with knee osteoarthritis (OA). This benefit was seen at short- to midterm follow-up.
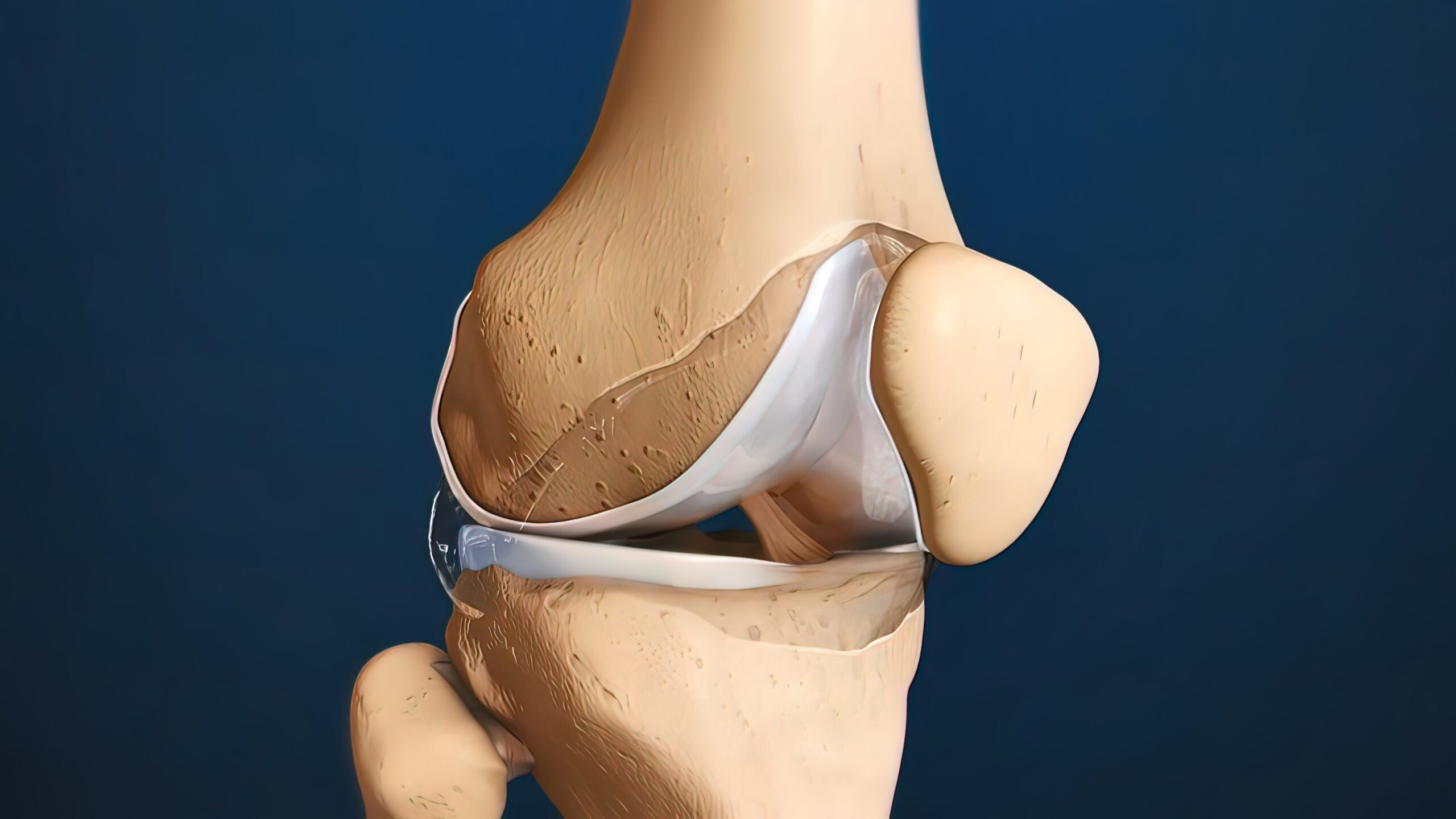
Cartilage Defects
Research studies have shown that BMAC injections are effective in treating cartilage injuries or defects of the cartilage. In some cases this can be combined with cartilage restoration procedures or performed as an isolated injection.
Sports Injuries and Fractures
BMAC has been used to help fractures heal more quickly. A recent systematic review and meta-analysis in the American Journal of Sports medicine suggested that using BMAC to augment surgical fixation of a common sports foot fracture (Jones fracture) resulted in higher healing rates of the fracture.

Why Choose Us?
Reviews of Dr. Burnham and Team
"Dr. Burnham and his staff are amazing. They have been extremely patient and accommodating to me through many personal issues impacting my knee surgery and recovery. They do not rush you during your office visit, and are thorough and answer all questions. "

Anonymous
Verified Healthgrades Review
Individualized, patient-centered approach
Every patient is unique. Our team works together to develop a patient-focused treatment plan. There is no "one-size-fits-all" surgery.
BOARD CERtified & Fellowship-trained
Dr. Jeremy Burnham is certified in Orthopedic Surgery and in Orthopedic Sports Medicine by the American Board of Orthopedic Surgery (ABOS).
Multi-disciplinary sports medicine team
Dr. Burnham works with a multi-disciplinary team who shares the common goal of using the most evidence-based treatment options to get you back to your sport or activity. His team is located in Baton Rouge, Louisiana.
As Medical Director of Sports Medicine at Ochsner Baton Rouge, Dr. Jeremy Burnham brings elite-level orthopedic care to athletes of all levels. His pioneering work in complex knee reconstruction and ACL surgery has established him as a trusted leader in sports medicine throughout the Gulf South.
Combining cutting-edge research as site investigator for NIH and Department of Defense initiatives with his roles as team physician for Southern University, the Baton Rouge Rougarou, and local high school programs, Dr. Burnham's evidence-based approach ensures every patient receives innovative treatment tailored to their recovery goals.

Faq.
BMAC stands for "Bone Marrow Aspirate Concentrate." It includes numerous factors from the spongy tissue within the bone known as the bone marrow. It includes mesenchymal stem cells, regenerative cells, regenerative growth factors, anti-inflammatory cells, and more.
After it is collected through aspiration from the bone, it is centrifuged, and the concentrated fluid is then applied to the injured or damaged tissue. Sometimes it is used in conjunction with some type of surgical repair, and other times it is used as an isolated injection.
BMAC stands for "Bone Marrow Aspirate Concentrate."
After obtaining the BMAC, it can be used for a variety of treatments. Often times it is injected into the knee, shoulder, or hip to treat osteoarthritis, cartilage damage or cartilage defects, meniscus tears, or other joint injury or joint pain. This can be done under x-ray guidance or with ultrasound to ensure the injection is targeting the correct location.
It can also be utilized as an addition to surgical repair of fractures or surgical repair of tendons such as rotator cuff repair, ACL reconstruction or ACL repair, MCL repair, and more.
BMAC Recovery Procedure: The recovery time after BMAC is usually very quick. Patients typically feel soreness at the aspiration site for 2-3 days. The positive effects of the procedure usually take 6-8 weeks to notice.
Patients can usually slowly resume normal activity after 2-3 days. Exercise can resume at 1-2 weeks, but cutting your normal exercise volume in half to start.
Serious side effects are uncommon. However, risks include hematoma formation at the aspiration site, or soreness at the aspiration or injection site. Less common risks would include infection, persistent pain, and persistent bleeding.
Ice and pressure should be applied after the procedure. It is recommended to keep the aspiration site covered and avoid submersion or getting it wet for 48 hours.
BMAC and PRP are two separate things, but are often used to treat similar conditions. PRP stands for "platelet rich plasma" and is obtained from peripheral blood (usually obtained from a blood draw in the arm). BMAC is obtained from the bone marrow. However, there are some similarities in growth factors and anti-inflammatory cytokines between the two. Both are considered "orthobiologics."
Make sure and follow your physician's instructions. Most of the time you will be asked to stop antiinflammatory medications or NSAIDs for a short time before and after the procedure.
We also recommend eating a balanced diet and hydrating well with plenty of water in the days leading up to the procedure. You will likely need someone to drive you to and from the procedure.
BMAC is not currently covered by insurance. This treatment is considered "investigational/experimental" by insurance plans. These services are usually offered on a self-pay basis.
The device used to process BMAC is cleared by the FDA. The BMAC itself does not currently require FDA approval because it is made using your own cells, for homologous use, and with minimal manipulation. It is not considered a drug and BMAC is one of the only FDA-approved delivery methods for mesenchymal stem cells.
BMAC is one of the most commonly studied orthobiologic procedures in regenerative medicine space. The specific procedures in orthopedics have not been FDA approved for tendons, ligaments, cartilage, joints, or muscles. The procedures described are based on “firm scientific rational and on sound medical evidence” and consistent with FDA guidelines. The use of BMAC has some of the most safety data behind its use, and is generally considered an accepted practice.
Treatment is described in the mentioned studies are consistent with the 2017 FDA Guidance Document on Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-Based Products: Minimal Manipulation and Homologous Use. Please see FDA recommendations about unregulated stem cell use.








